Blog
Welcome to the section of Sabal Food Safety Consulting's web page dedicated to brainstorming discussions about food safety and related topics. Feel free to leave your comments or opinions about the topics under discussion. Should you enconter any difficulty using the Blog, send us an email to This email address is being protected from spambots. You need JavaScript enabled to view it. so we can investigate the problem.
Over here you'll see all publicly shared articles. Should you want to leave a comment on any article, click on the title and another window will open with the article and, at the bottom, there is a section where you can share your comments.
You could also register as a member and you'll receive notifications when new articles are published and, you'll have access to the articles developed only for registered members. Registration is free.
It is nice to have you here!
Food and drugs are edible

Food and drugs are edible. What’s the difference between them?
We can see everywhere the promotion of “edible cannabis”, CBD oils, HEMP edibles and other terms promoting the consumption of products derived from cannabis plant as food and claiming they treat medical conditions. I performed an investigation into some of these topics trying to better understand what they mean.
What does “edible” mean?
“Something that is suitable or safe to eat”. Source: Merriam-Webster Dictionary.
Assuming this is an acceptable definition of “edible”, both food and drugs can be categorized as edible.
What does “food” mean?
Based on the US Laws, food means: (1) articles used for food or drink for man or other animals, (2) chewing gum, and (3) articles used for components of any such article (Ingredients). Source: 21 USC 321(f).
The Food and Agriculture Organization (FAO) define “Food Security” as: “Food security exists when all people, at all times, have physical and economic access to sufficient, safe and nutritious food that meets their dietary needs and food preferences for an active and healthy life”. Source: World Food Summit, 1996. The same source indicates: “It is the nutritional status of the individual household member that is the ultimate focus” and, “The definition of sub-nutrition includes poor absorption and/or poor biological use of nutrients consumed”
By reading the previous definitions from US and international sources, we can infer that food is eaten for nutritional purposes; to nurture our bodies for “an active and healthy life”.
What does “drug” mean?
In US Law, a drug is “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals”.
After reading this, it is clear that a drug is not food. A drug is related with the healing of the body, not for nutritional purposes. Note that the definition of drug explicitly excludes food.
The World Health Organization (WHO) has three definitions for a drug. “In medicine, it refers to any substance with the potential to prevent or cure disease or enhance physical or mental welfare, and in pharmacology to any chemical agent that alters the biochemical or physiological processes of tissues or organisms.” However, “In common usage, the term often refers specifically to psychoactive drugs and often, even more specifically, to illicit drugs, of which there is non-medical use in addition to any medical use.” WHO indicates in this definition that “caffeine, tobacco, alcohol and other substances in common non-medical use are also drugs in the sense of being taken at least in part for their psychoactive effects.” Source: World Health Organization, Lexicon of Alcohol and Drug Terms, 1994.
What does “nutrition” mean?
The act or process of nourishing or being nourished. Specifically: the sum of the processes by which an animal or plant takes in and utilizes food substances. Source: Merriam-Webster Dictionary.
We eat for nutritional purposes. Food products are meant for nutritional purposes. There are some drugs that are allowed to be consumed as food, like alcohol, tobacco, coffee and their use is strictly regulated.
What about HEMP and Cannabis?
On April 12th, 2018, a bill was introduced into the US Congress to amend the Agricultural Marketing Act of 1946. This Act may be cited as “Hemp Farming Act of 2018”. Hemp is defined as “…the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.”
On December 20th, 2018 the “Hemp Farming Act of 2018” was signed into Law. On that date, the FDA Commissioner released a statement titled “Statement from FDA Commissioner Scott Gottlieb, M.D., on signing of the Agriculture Improvement Act and the agency’s regulation of products containing cannabis and cannabis derived compounds”. The following notes were extracted from it (In quotes all notes from the FDA’s statement):
“Hemp was removed from the Controlled Substances Act, which means that it will no longer be an illegal substance under federal law.” This means that there are substances that are illegal and cannot be used for any purpose, including food or drugs.
“The FDA preserves the regulatory authority over products containing cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic Act (FD&C Act). This allows the FDA to continue enforcing the law to protect patients and the public while also providing potential regulatory pathways for products containing cannabis and cannabis-derived compounds.”
“We continue to be concerned at the number of drug claims being made about products not approved by the FDA that claim to contain CBD or other cannabis-derived compounds. The FDA requires a cannabis product (hemp-derived or otherwise) that is marketed with a claim of therapeutic benefit, or with any other disease claim, to be approved by the FDA for its intended use before it may be introduced into interstate commerce.” In this case, the product is a drug.
“It’s unlawful under the FD&C Act to introduce food containing added CBD or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp-derived. Under the FD&C Act, it’s illegal to introduce drug ingredients like these into the food supply, or to market them as dietary supplements. It should also be noted that some foods are derived from parts of the hemp plant that may not contain CBD or THC, meaning that their addition to foods might not raise the same issues as the addition of drug ingredients like CBD and THC.” So, as long as there is evidence that CBD or THC are not present in the food product, the FDA will allow its use as food.
“The FDA will continue to take steps to make the pathways for the lawful marketing of these products more efficient. These pathways include ways for companies to seek approval from the FDA to market with therapeutic claims a human or animal drug that is derived from cannabis”
“We are announcing that the agency has completed our evaluation of three Generally Recognized as Safe (GRAS) notices related to hulled hemp seeds, hemp seed protein and hemp seed oil and that the agency had no questions regarding the company’s (the company that introduced the GRAS notices for those three products) conclusion that the use of such products as described in the notices is safe. Therefore, these products can be legally marketed in human foods for these uses without food additive approval, provided they comply with all other requirements and do not make disease treatment claims”.
Conclusion
The only three products derived from Hemp that can be used as food are: hulled hemp seeds, hemp seed protein and hemp seed oil. The products cannot contain CBD or THC and cannot make any claim to treat any disease. Only under these conditions, in the US, it is possible to talk about food safety related to hemp.
However, we should also consider how any food product derived from cannabis, not containing CBD or THC, will nurture our bodies. Which are the nutrients provided to maintain “an active and healthy life” like the World Health Organization indicates? Why would one eat something that may not contribute to the nutrition of the body?
The future may bring evidence supporting the potential nutritional content of CBDs on the body, or not.
What is experience?

Opinion: What is experience?
Based on Merriam-Webster’s dictionary, the definition of experience is: a) direct observation of or participation in events as a basis of knowledge; b) the fact or state of having been affected by or gained knowledge through direct observation or participation; c) practical knowledge, skill, or practice derived from direct observation of or participation in events or in a particular activity; d) something personally encountered, undergone, or lived through; e) the act or process of directly perceive events or reality.
Understanding what “experience” means seems easy. However, let’s take a deeper analysis of this word and its meaning.
Two or more people looking at the same event will have different reactions to it and this depends on their personality and knowledge. This leads us to conclude that the experience of each person depends on what is perceived, analyzed and kept after observing an event.
There are a few methods to evaluate the personality of people. One of them identifies the following blocks of attributes to create profiles: introvertive or extravertive, sensitive or intuitive, thinker or emotional and, judging or perceptive. Based on “CarrrerPlanner.com”, 13.8% of the US population belongs to the group of people that are “introvertive, sensitive, emotional and judging” (ISEJ). Based on the same source, the percentage of US population that belongs to the group of people that are “extravertive, intuitive, thinker and perceptive” (EITP) is 3.2%. In theory, this last group will achieve consistent outcomes when they have experience in something.
Now, imagine an ISEJ and an EITP persons exposed to the same event. The experience of these two individuals, most likely, will be different as it will depend on their personal attributes.
Based on a TedX presentation by Victor Kuppers about the attitude of people, there is a way to determine the “value” of a person. This “formula” suggests that the value of a person depends on his/her knowledge, skills and attitude. By using this formula and concept, two people receiving the same training for the same activity, using the same training material, at the same time and the same trainer will perform differently during the execution of such activity. Victor indicates that the attitude plays a significant role in the outcome of the execution of the activity. This attitude is related to the personality of each individual and it directly affects the experience.
I believe it is important to recognize that, as part of the definition of experience, there is something that is not intrinsically implicit within its definition. When someone performs an activity, it is expected that its outcome will achieve whatever was the intention of it. This intention is typically related with science, common sense or any other criterion/criteria that will validate the outcome of such activity. When defining experience, we assume that the person performing an activity has the right knowledge, skills and attitude that allows him/her to achieve the expected outcome consistently, validated by the criterion used during the creation of the activity. This is “valid experience”.
Assuming the previous explanation is applicable to a population, a small percentage of people will have the experience necessary to perform the activities assigned to them consistently and obtaining results that are in compliance with the criteria used to validate them.
As a final note, it is possible to have many years of experience doing things wrong or partially wrong. How many times we had attended meetings where the host asks each attendee for the number of years of experience to add them all and show the accumulated years of experience of the group? Assuming that 3.2% of a population has the best combination of personal attributes, the right number of years of experience will be obtained by multiplying the sum of all those years by 0.032. In other words, in a group of 100 people, the number or years of “valid experience” is retained only by about 3 persons of the group.
System's Consistency & Continuous Improvement

www.sabalfsc.com
Consistency and continuous improvement of a system
Whenever a decision is made to implement a system, the expected outcome is to achieve consistency on whatever will be done, the purpose. The system must be able to attain its purpose consistently.
I learned many years ago that, when developing systems, what is critically important is to make sure we comply with the requirements identified, which could be mandatory (legal requirements) or voluntary (like following an ISO Standard). This is what I believe is the most difficult aspect during the development of the system. This is understanding what we are trying to control and how we are going to do it, all in compliance with the requirements.
Understanding what we are trying to control is the first step. No matter what sector of the industry a system will be developed and implemented for, the “hazards” or “not desirable conditions” to the finished product or service must be adequately identified and understood, as well as knowing their sources. This should allow us to develop procedures that will effectively control them.
Now, some of the requirements may not be related with hazards or conditions we are trying to control but with the systems’ management components. As an example, making senior management aware of the overall effectiveness of the system is a common requirement in any system, with the intention to make them aware of the overall operations and potential needs for resources to maintain consistency and/or to improve the system.
In theory, when a system is developed, the intention is to design it to be effective all the time. That’s why it’ll be necessary to make sure that all elements, individually, and the system as a whole will achieve the purpose of the system.
It’s a fact that systems do not operate as intended all the time and deviations will occur. Whenever a system deviates from the requirements being followed it is not effective anymore. Therefore, all systems must include, as one of the its management components, procedures on how to deal with deviations, commonly known as corrective and preventive actions.
These corrective and preventive actions’ procedures have a very particular and unique purpose, to bring the system back into compliance with the requirements, which is the definition of a corrective action and, to do something to prevent the cause of the deviation from happening again, which is the definition of a preventive action. Corrective and preventive actions’ procedures are critically important to be well understood within the purpose of a system as they are the only resource a system has to recover from a deviation, including dealing with the affected product or service and, to prevent the reoccurrence of whatever caused the system to deviate from its purpose.
Corrective and preventive actions’ procedures are not related with the hazards or conditions controlled by the system. They are related with the assurance that the system is achieving the intention of the requirements and its overall purpose. Proper implementation of these procedures will provide the system with continuous improvement.
Feel free to contact us should you want to develop or improve your food safety system at This email address is being protected from spambots. You need JavaScript enabled to view it. .
Legal and Regulatory Compliance

May 22nd, 2018
Recently, I received an email asking for guidance on how to approach the effectiveness of the cleaning procedures in a bakery were conveyor belts made of a cloth-type material are still used. I would like to share the approach I provided in my answer...
Any company in the US, and probably in any country of the world, MUST be in full compliance with all applicable legal and regulatory requirements before thinking in getting involved in following any voluntary scheme like those benchmarked by GFSI. It is just NOT acceptable to be in full compliance with any of these schemes and not being in compliance with the laws and regulations applicable to the activities performed by the company when manufacturing food products.
Since June 30th, 1906, the definition of “adulteration” can be found in Subchapter I of the Federal Food and Drug Act (FFDCA). It “made it unlawful to manufacture adulterated or misbranded foods or drugs in Territories or District of Columbia and provided penalty for violation.”
The last amendment of the definition of adulteration in the Federal Food, Drug and Cosmetic Act of 1938 was made on October 1st, 2005. The definition of adulteration in the Law grew to include other conditions that will render the food products adulterated. As defined in Section 348 of the FFDCA, “A food additive shall, with respect to any particular use or intended use of such additives, be deemed to be unsafe for the purposes of the application of clause (2)(C) of section 342(a) (adulteration) of this title, unless...(3) in the case of a food additive as defined in this chapter that is a food contact substance, there is: (A) in effect, and such substance and the use of such substance are in conformity with, a regulation issued under this section prescribing the conditions under which such additive may be safely used".
Cloth-type food contact surface does not comply with the above requirement, period!. Moreover, 21 CFR 117.40 - Equipment and Utensils, in Subpart B - Good Manufacturing Practices, states: "All plant equipment and utensils used in manufacturing, processing, packing, or holding food must be so designed and of such material and workmanship as to be adequately cleanable, and must be adequately maintained to protect against allergen cross-contact and contamination".
So, whomever designed and sold a cloth-type conveyor equipment was not knowledgeable of the laws and regulations, as well as those companies that purchase that type of equipment. Only those that purchased and used this type of equipment are in violation of the Law.
It doesn't matter if third or second party auditors have seen that type of equipment working for centuries and accepting them. This type of equipment, since 1906, will produce food products that are, by definition of the Law, adulterated.
It is time for everyone in the food industry to start reading, in detail, or looking for help outside the company to understand legal and regulatory requirements as this is what will be used in the very near future by the FDA when performing their investigations. They will NOT look to see if you are in compliance with the requirements of the GFSI scheme the company decided to follow voluntarily! You might have a perfect score in your GFSI based audit and receive a Form 483 and/or a warning letter from the FDA for lack of compliance with all legal and regulatory requirements.
FSPCA Certificate - Printing or downloading instructions

Procedure to download or print your FSPCA Certificate
Note: The pictures may take some time to load depending on the speed of your connection to the internet. Please, allow some time for them to load.
Step 1 - You’ll be receiving an email from the “International Food Protection Training Institute” ( This email address is being protected from spambots. You need JavaScript enabled to view it. ). Be vigilant as the email may fall in your junk email folder. The email looks like the picture below.
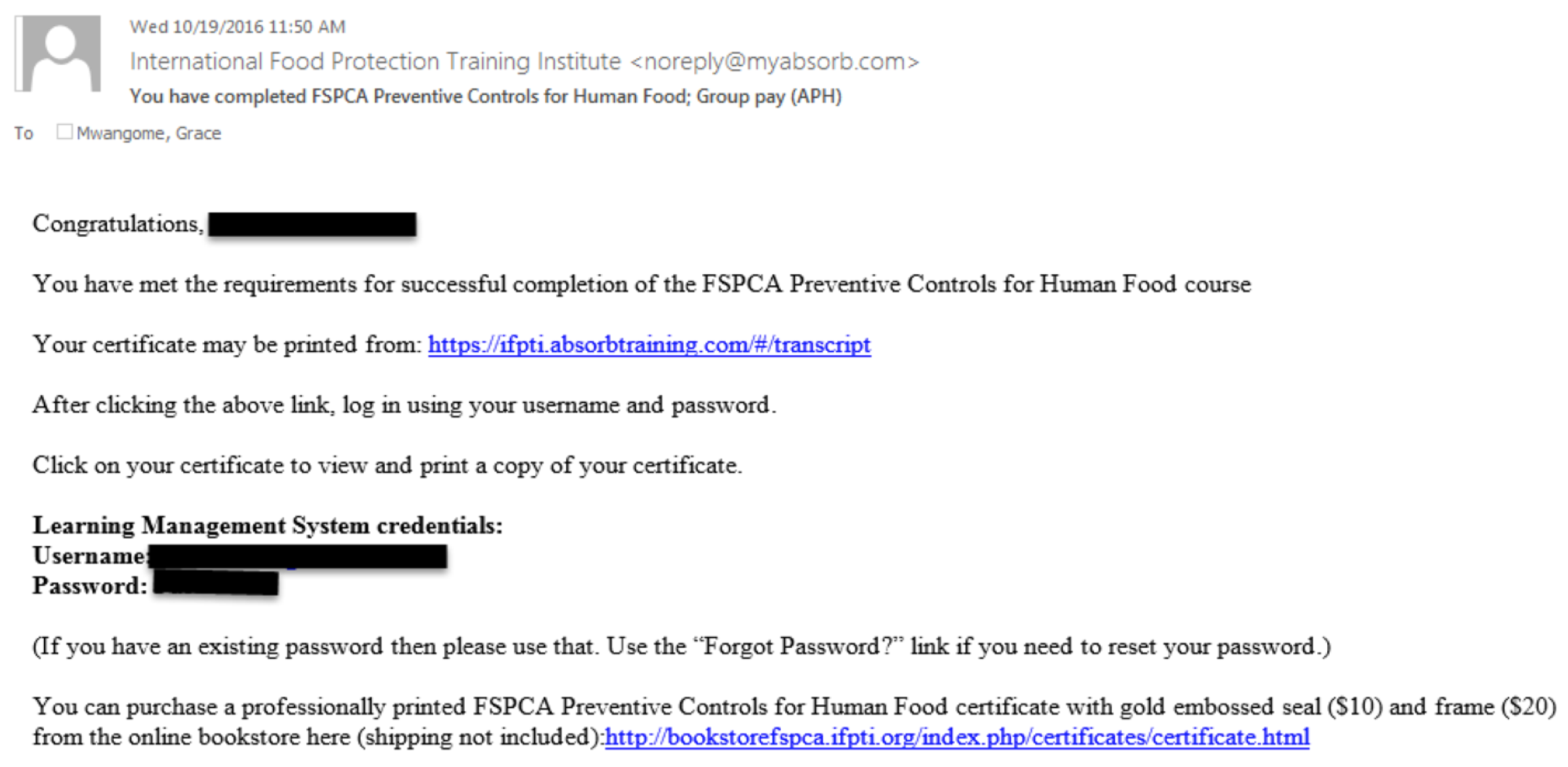
This email has a link (https://ifpti.absorbtraining.com/#/transcript) to the webpage that you'll be accesing to print your certificate. Also, the email contains the username and password that you will be using to sign-in after the webpage opens.
When you click the link in your email, the following webpage will open:
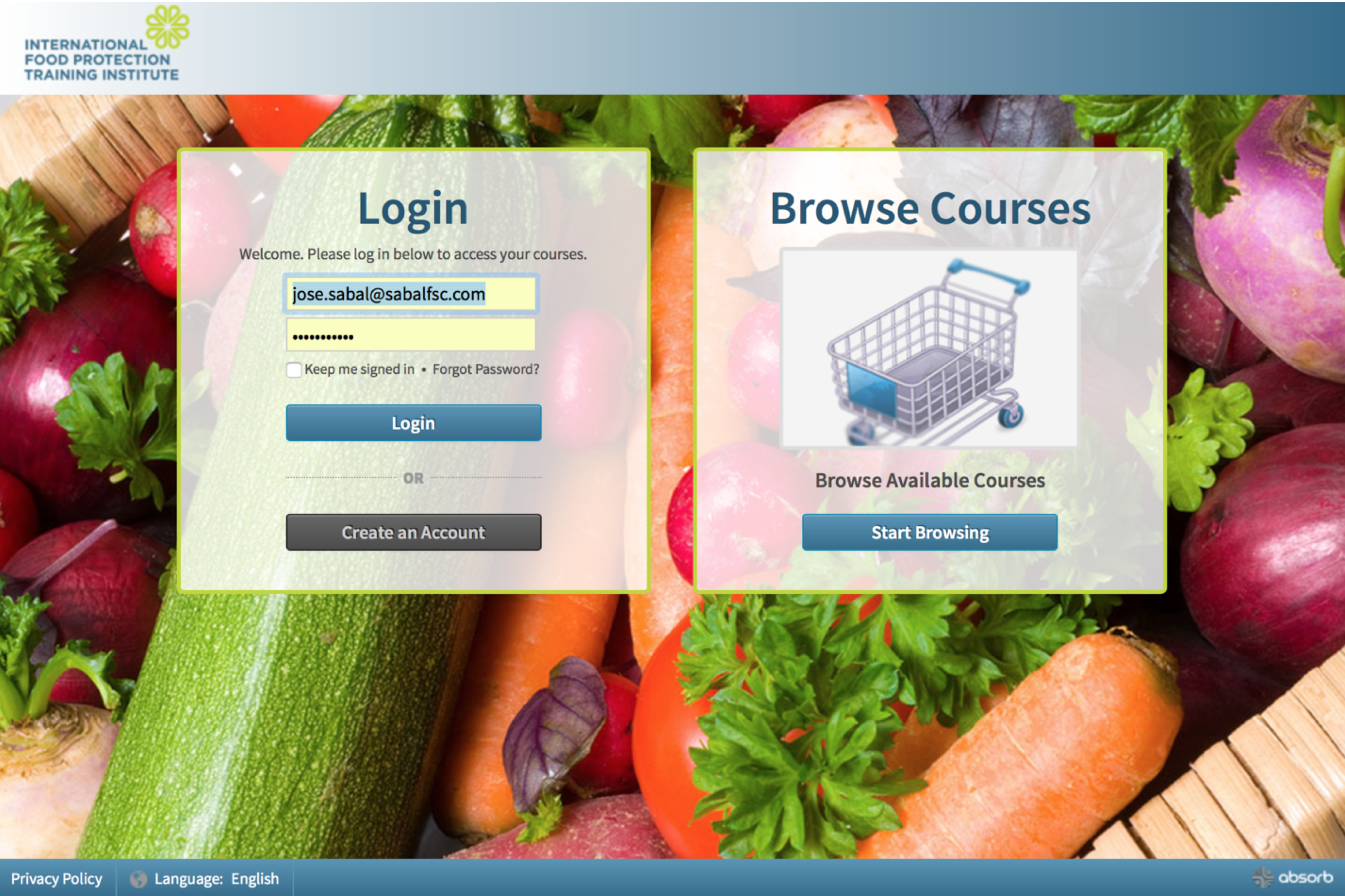
Step 2 - Populate the first field of the “Login” box with the username provided in the email. Populate the second field with the password provided in the email. Be careful as the password is case sensitive.
Properly populating the fields will take you to the following page:
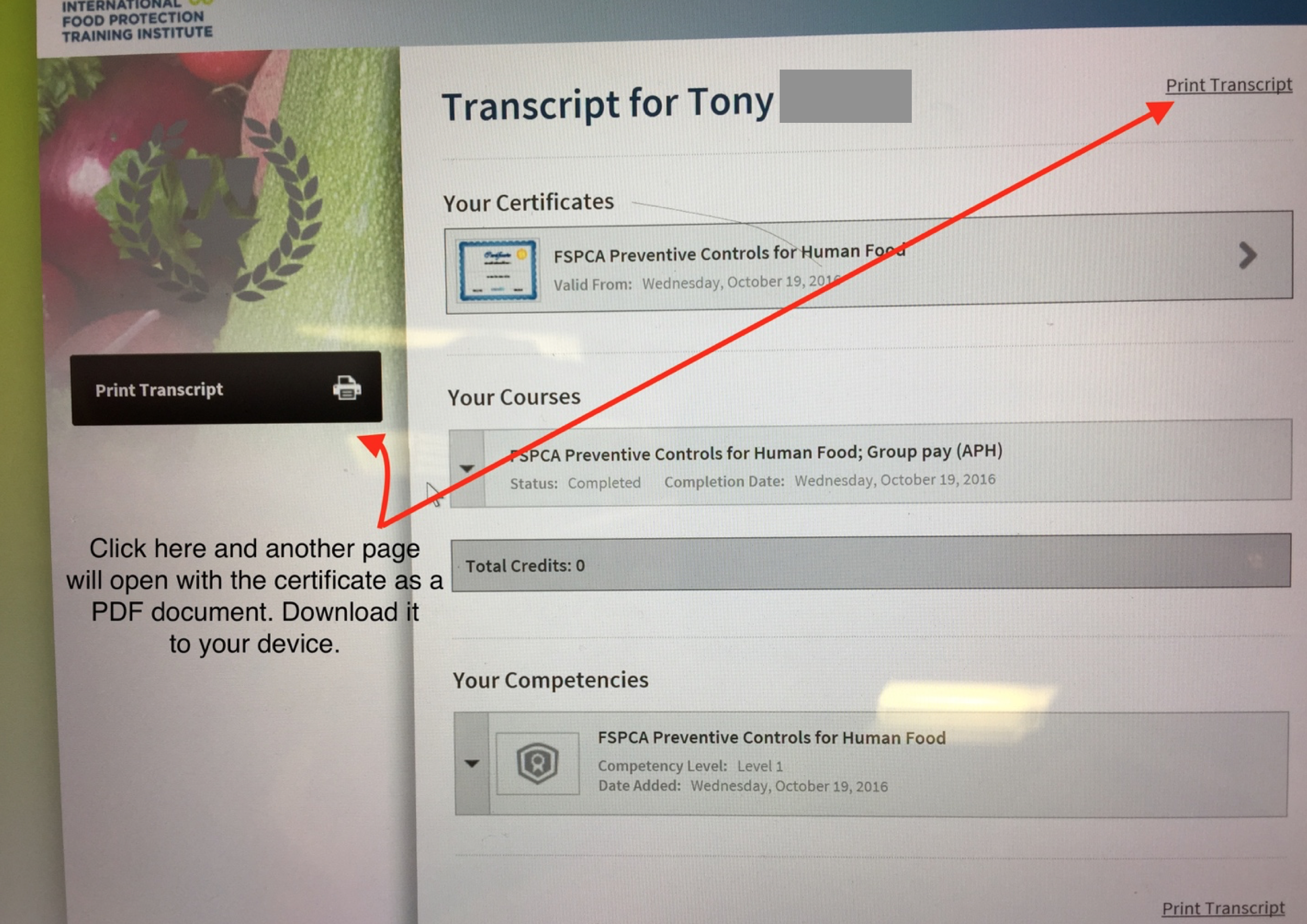
Step 3 - This page has two sections that can be clicked to “Print Transcript”. By clicking any of the two, another page will open with the certificate in PDF. Download it to your computer and/or print it.
If for any reason this does not work, contact your trainer or the training center.
Best regards,
Jose Sabal




